Abstract
INTRODUCTION: T-lineage acute lymphoblastic leukemia (T-ALL) and its more immature subset, early T precursor (ETP) ALL, have limited treatment options after first-line chemotherapy. New treatment approaches are necessary for this high-risk group, as well as for older adults who may be unfit for intensive pediatric-inspired regimens. T-lymphoblasts depend on BCL-2 family of antiapoptotic proteins for survival and targeting these proteins with new BH3 mimetics may improve outcomes. A phase 1 study of venetoclax (BCL-2 inhibitor) plus navitoclax (BCL-2/BCL-XL inhibitor) and chemotherapy resulted in a complete remission rate of 53%, considerably higher than historic controls. The dose-limiting toxicity of navitoclax is thrombocytopenia, which can potentially be overcome by LP-118, a next-generation BCL-2/BCL-XL inhibitor. LP-118 has a modified structure with fine-tuned BCL-XL activity to minimize platelet toxicity. LP-118 has also demonstrated preclinical activity in hematologic malignancies harboring the BCL-2 Gly101Val mutation, which has been shown to drive venetoclax resistance. Given the significance of BCL-2 and BCL-XL pathways for survival of T-lymphoblasts, we set out to investigate the preclinical activity of LP-118 and other BH3 mimetics in T-ALL.
METHODS: Primary T-ALL and ETP-ALL patient samples were treated with BH3 mimetics in vitro for 48 hours and sensitivity to individual BH3 mimetics was determined. Additionally, BH3 apoptotic dependency profiling was performed with both plate-based and flow cytometric-based assays to determine mitochondrial depolarization and cytochrome c release, respectively. By using peptides specific for individual anti-apoptotic proteins, the cell death dependency patterns in samples pre- and post-treatment were also determined.
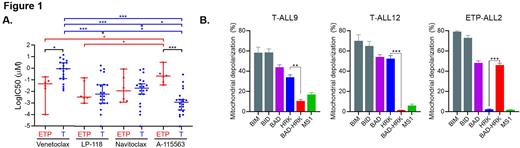
RESULTS: Sensitivity of T-ALL and ETP-ALL primary patient cells to cell death following treatment with different BH3 mimetics including venetoclax, LP-118, navitoclax, and A-1155463 (BCL-XL inhibitor) was first measured (Fig. 1A). Typical pre-T-ALL samples from 20 pts showed higher sensitivity to BCL-XL targeting (LP-118, navitoclax, A-1155463) when compared to BCL-2 targeting (venetoclax). In contrast, ETP-ALL samples from 6 pts showed higher sensitivity to BCL-2 inhibition- via BCL-2 (venetoclax) or dual BCL-2/BCL-XL inhibition. Interestingly, Western blot analysis revealed no correlation between the relative ratios of BCL-2:BCL-XL protein levels and response to different BH3 mimetics. Therefore, BH3 profiling was used to identify clinically meaningful selective antiapoptotic protein dependencies in these samples. Using this method, we observed a clear increased cell death dependency on BCL-XL in pre-T-ALL samples and upon BCL-2 in ETP-ALL samples (Fig. 1B). Combining surface immunophenotyping with flow-based BH3 profiling further allowed for evaluation of apoptotic dependencies in functionally heterogenous subpopulations of cells. Our data suggest that the CD34+CD7+ compartment may be enriched with cells that are less primed to die and more resistant to BH3 peptidomimetics.
CONCLUSIONS: Our data show maturation stage-specific differences in antiapoptotic protein dependencies in ETP- vs T-ALL, that correlate with their responses to individual BH3 mimetics. Based upon this work, we believe that dual targeting of BCL-2 and BCL-XL is a promising approach for further clinical development in both types of T-lineage ALL. Additionally, the dose limiting toxicity of myelosuppression observed with navitoclax may be overcome with the novel dual inhibitor, LP-118. The ongoing phase 1 study investigating oral LP-118 in relapsed/refractory hematologic malignancies is expected to provide data on safety and clinical activity in 2023 (NCT04771572).
Disclosures
Chen:Newave Pharmaceuticals: Current Employment. Tan:Newave Pharmaceuticals: Current Employment. Anthony:Newave Pharmaceuticals: Current Employment. Chen:Newave Pharmaceuticals: Current Employment. Shen:Newave Pharmaceuticals: Current Employment. LaBelle:AbbVie: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Stock:Pluristem: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; MorphoSys: Honoraria; Kura Oncology: Honoraria; Kite: Honoraria; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria; Servier: Honoraria; Syndax: Consultancy, Honoraria; Newave Pharmaceuticals: Consultancy; Agios: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal